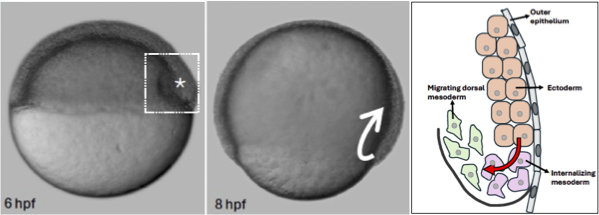
The zebrafish embryo: a model of vertebrate embryonic cell arrangement
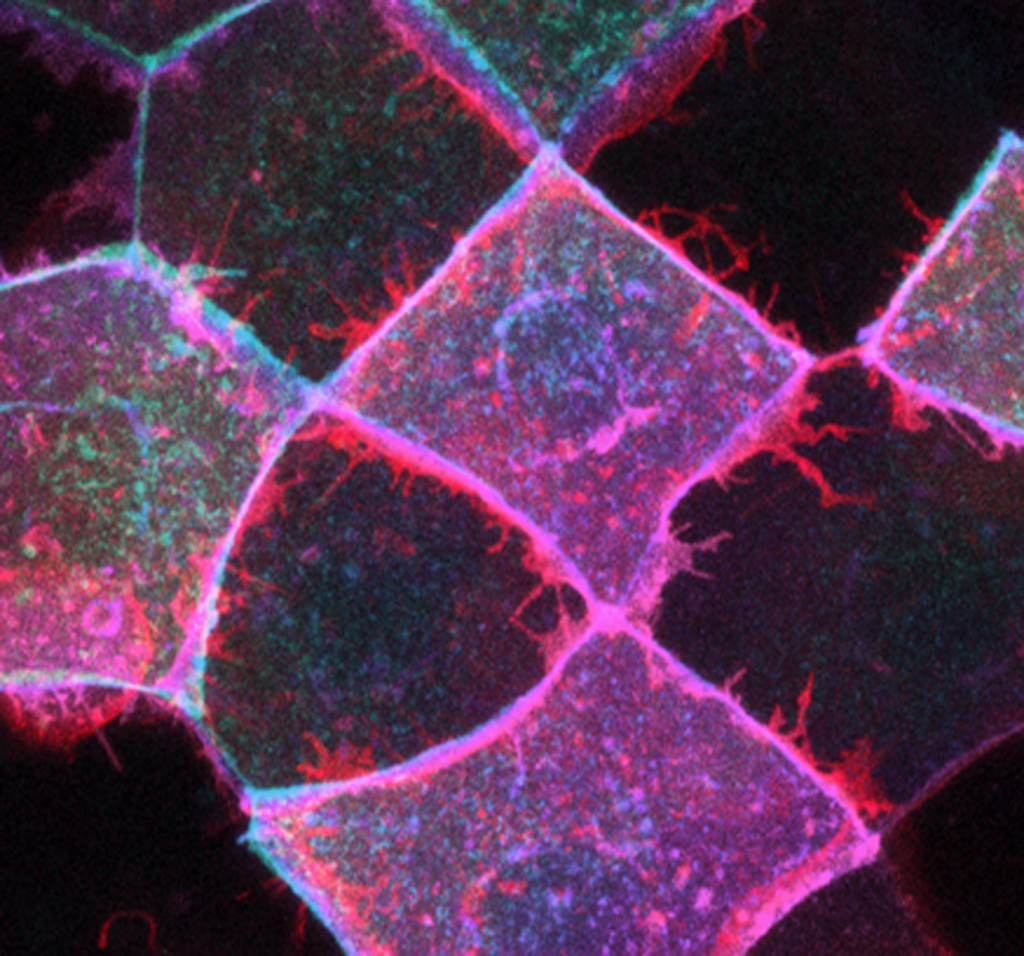
Epithelial morphogenesis: Epithelia undergo topological changes to maintain tissue homeostasis and drive morphogenesis. Actin networks play a central role in driving topological changes by remodeling cell-cell junctions both by shortening (contracting) and lengthening (stretching). We investigate the molecular mechanisms that enable the zebrafish embryonic surface epithelial sheet (the enveloping layer, EVL) to spread during tissue elongation. Focuses include the roles of the small GTPase Rab25 and, in collaboration with Tony Harris, the role of the Arf-GEF cytohesin.
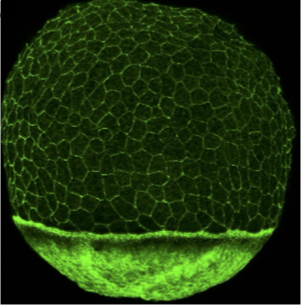
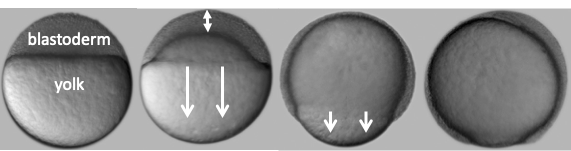
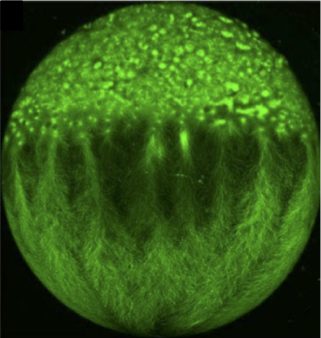
The role of the extraembryonic yolk cell in epiboly: The yolk cell generates the motive force for epiboly by assembling a contractile actomyosin ring in the yolk syncytial layer (YSL) (situated below in the EVL in the phalloidin image above). Large microtubule networks also encompass the yolk cell, and contribute to morphogenesis. We aim to understand the regulation of the actin and microtubules networks in the yolk cell. One focus is on the role of the microtubule minus-end binding protein Camsap2a.
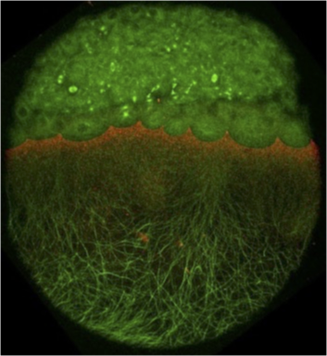
The role of Eph/ephrin signaling in tissue dynamics during zebrafish morphogenesis: Tissue morphogenesis can involve regional variations and transitions in tissue fluidity. Solid-like tissues exhibit abundant cell-cell contacts, few cell rearrangements and few interstitial gaps between cells, making the tissue resistant to shape changes. In fluid-like tissues, cells undergo widespread cell rearrangements, have reduced cell-cell contacts and more interstitial gaps and can easily remodel in response to internal or external forces. Tissue state transitions occur within the multi-layered zebrafish embryo model. We investigate the role of Eph/ephrin signalling in the molecular control of the highly reproducible spatiotemporal tissue state transition from solid- to fluid-like that occurs during dorsal mesoderm internalization in the zebrafish embryo.